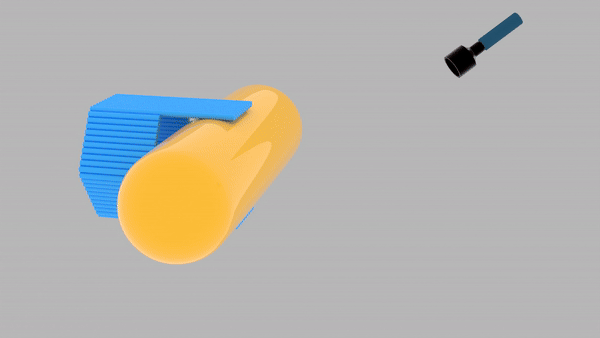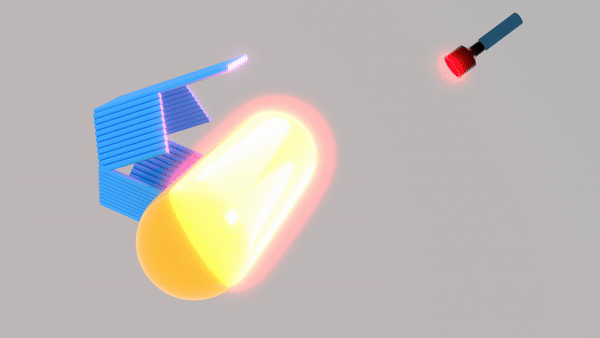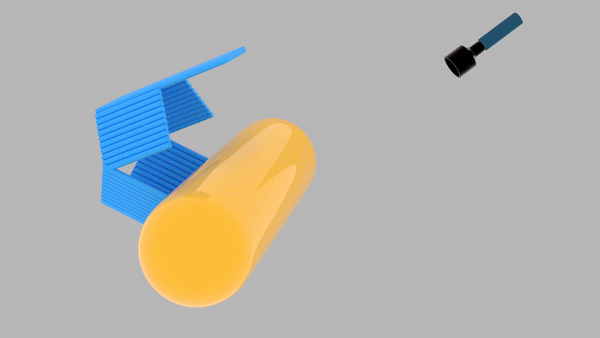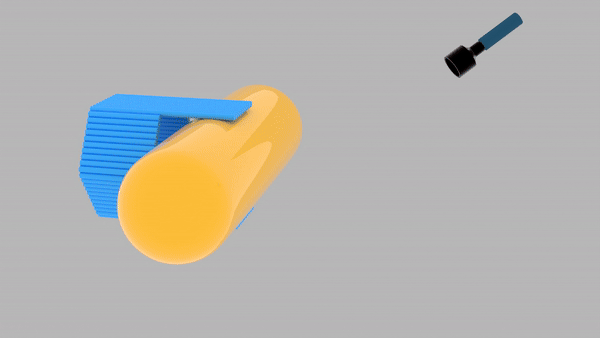
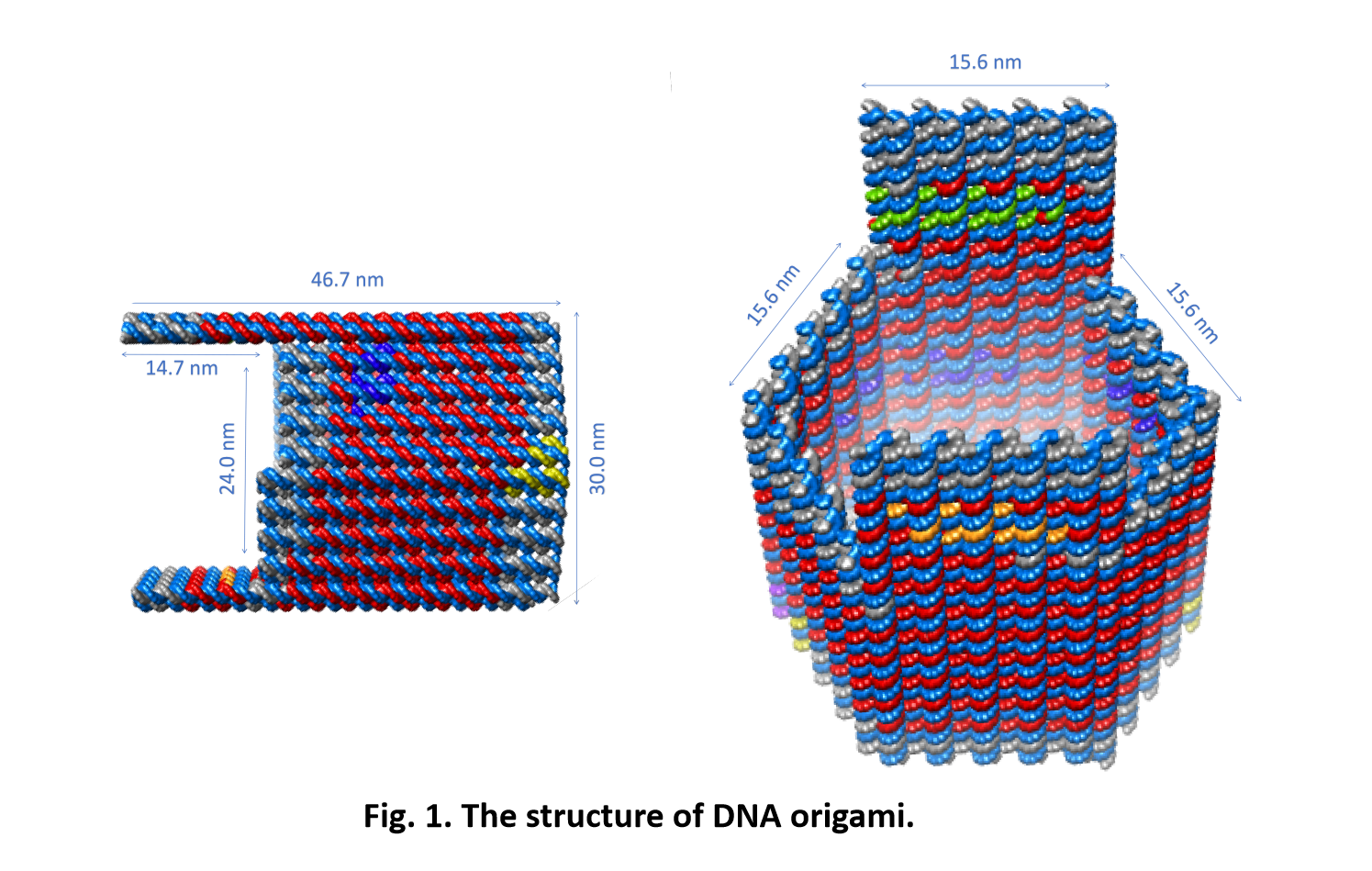
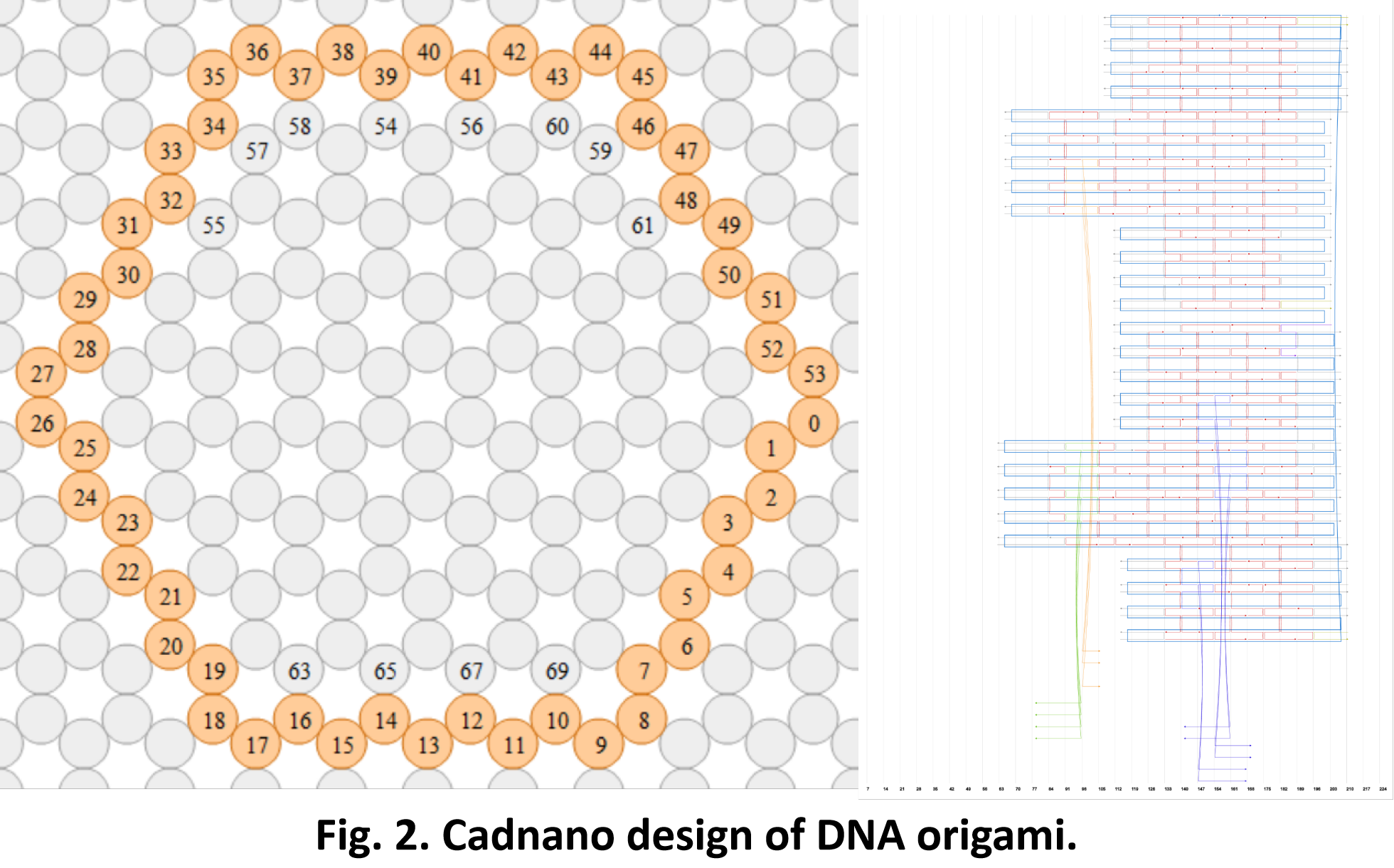
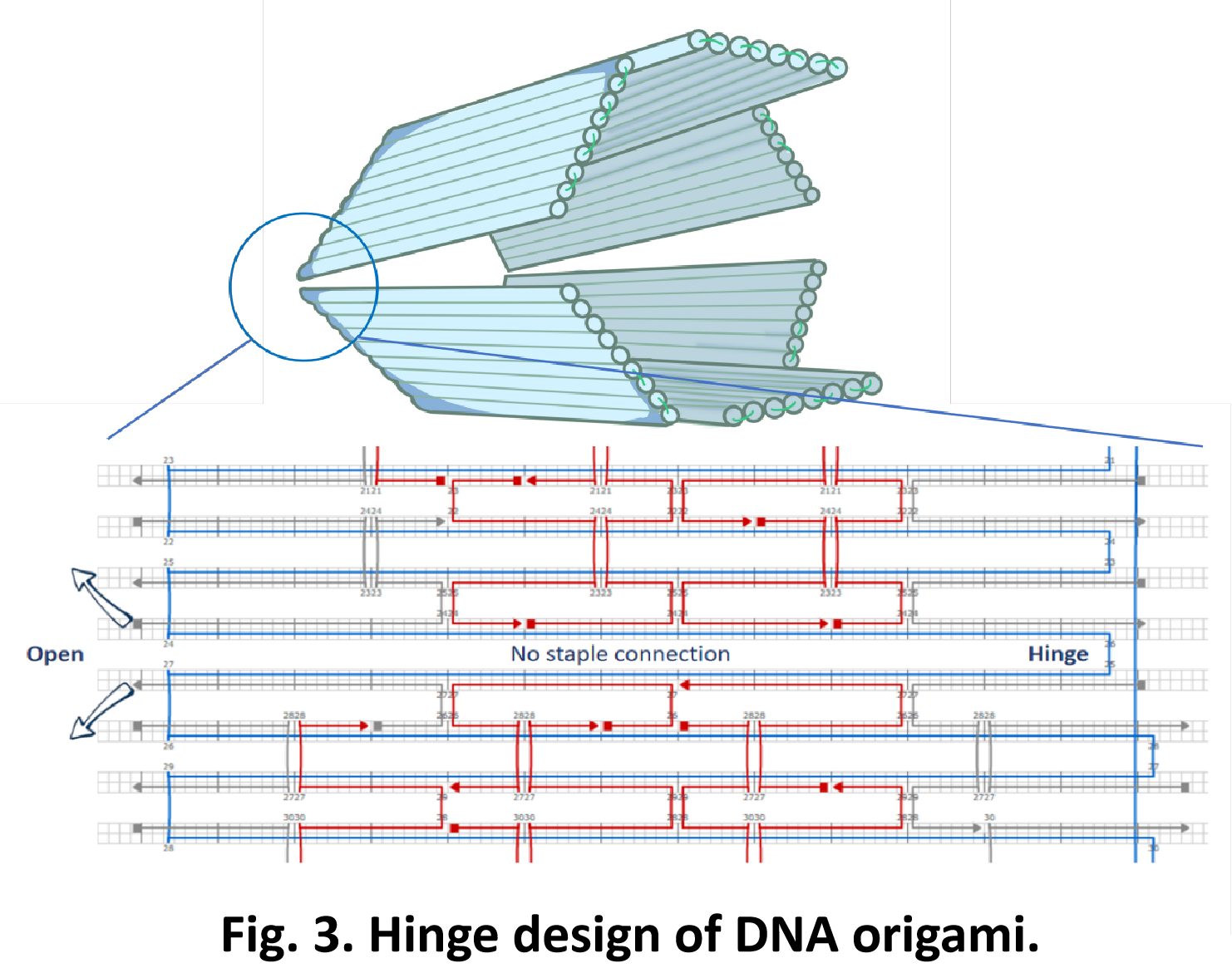
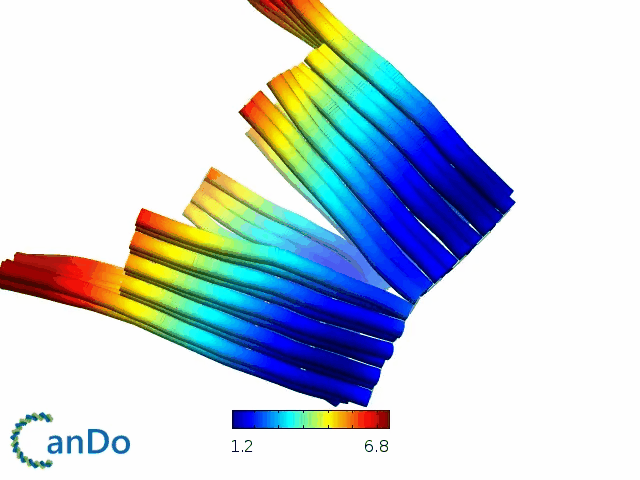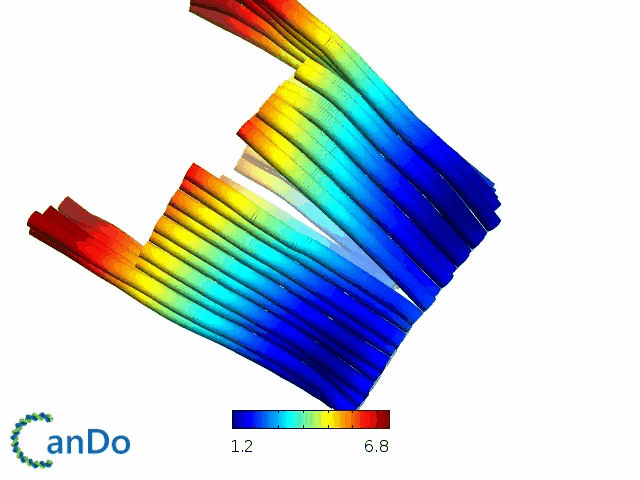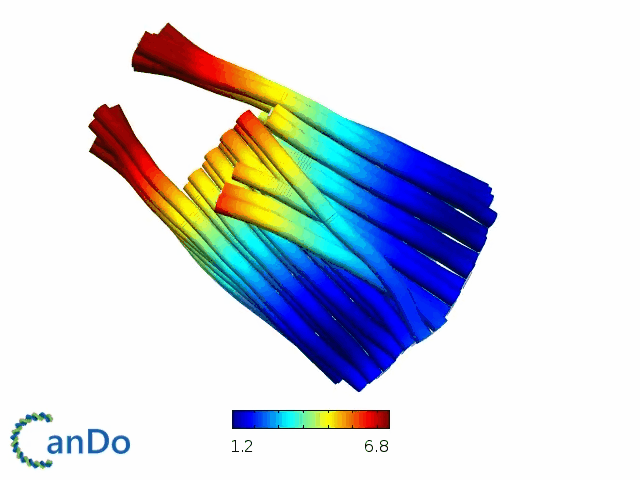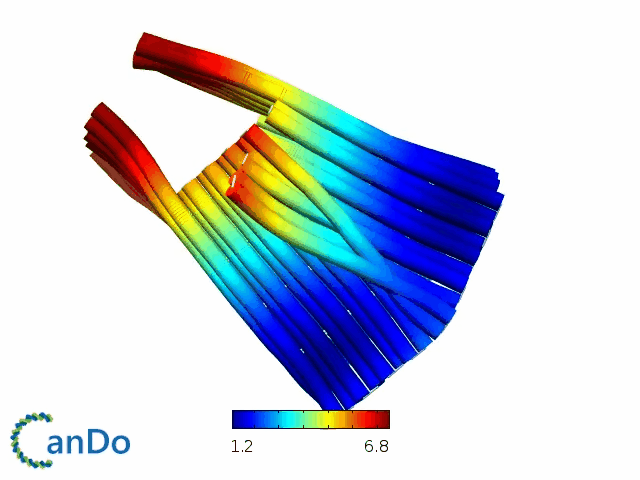
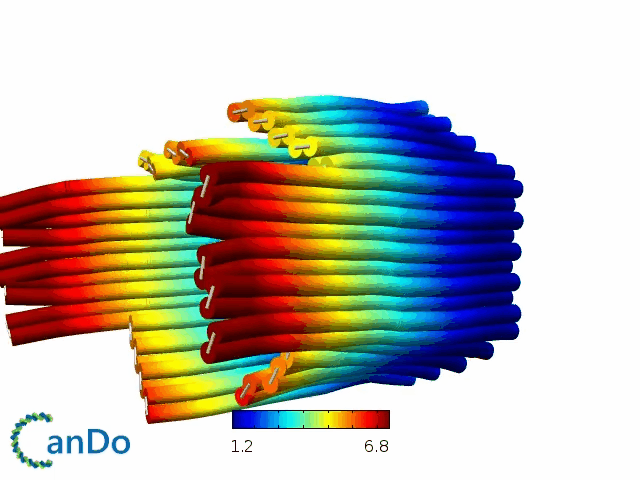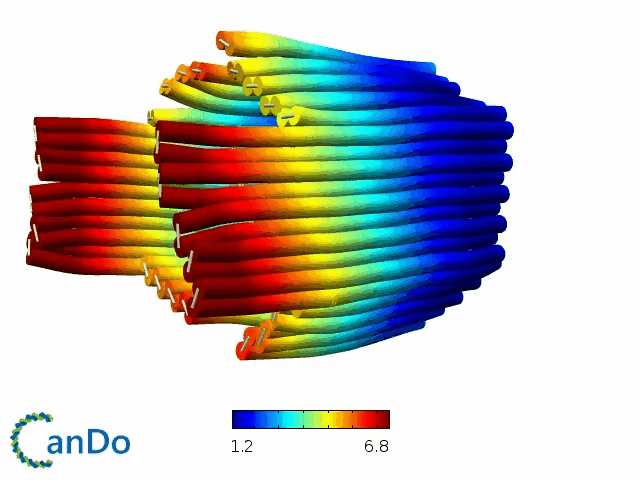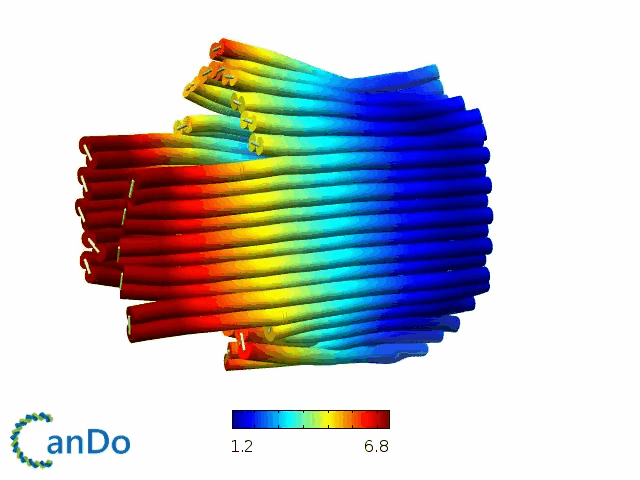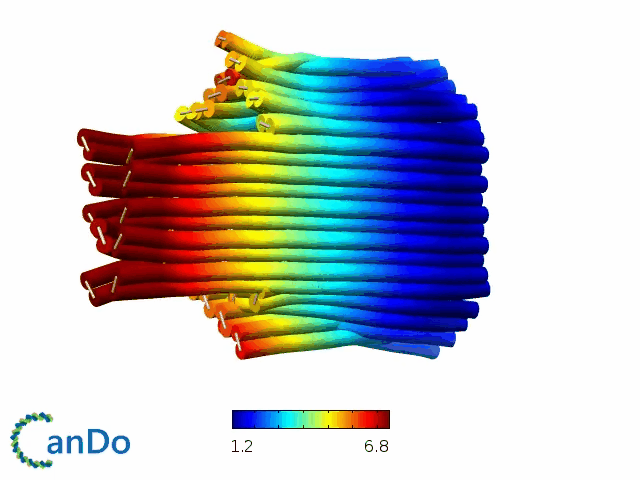
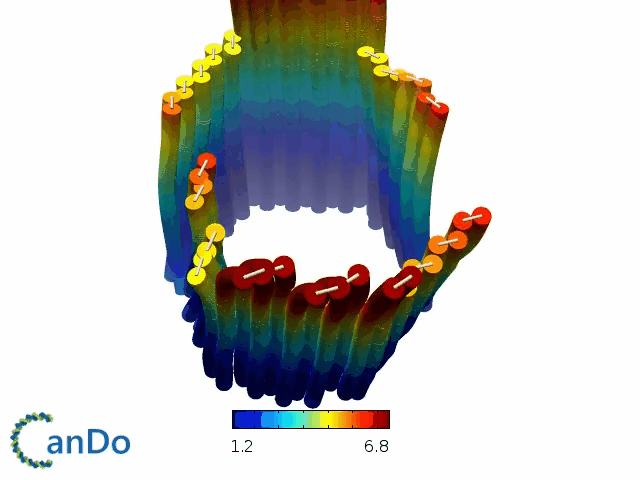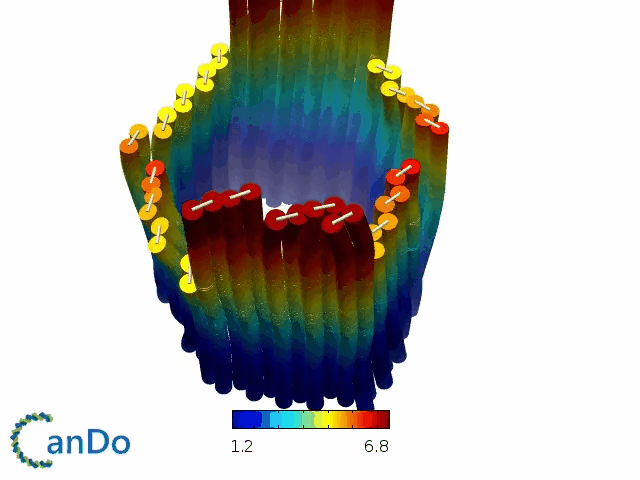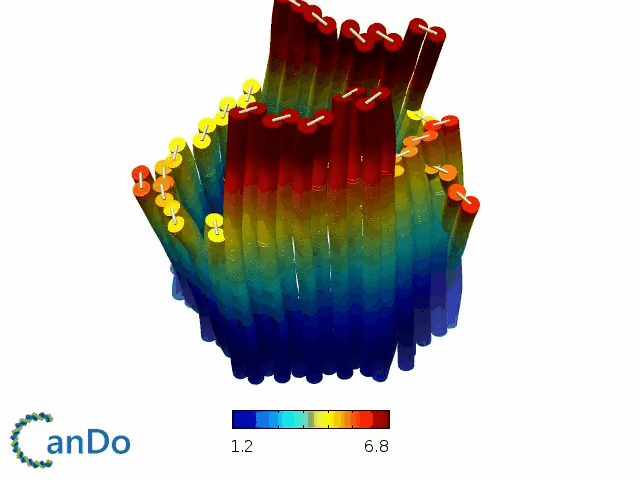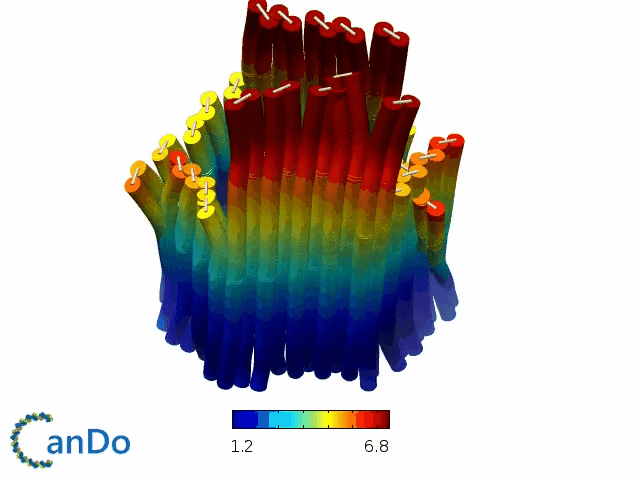
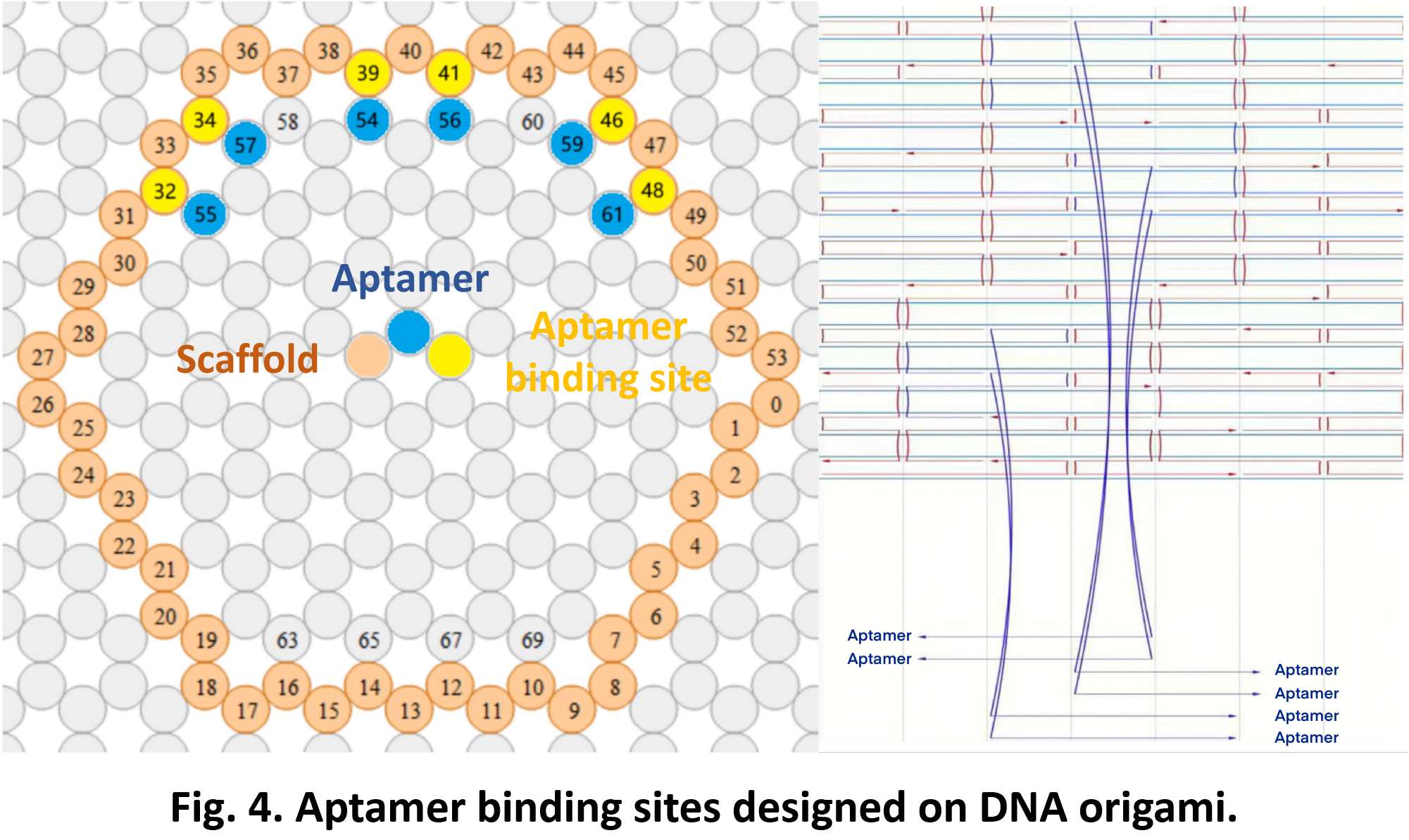
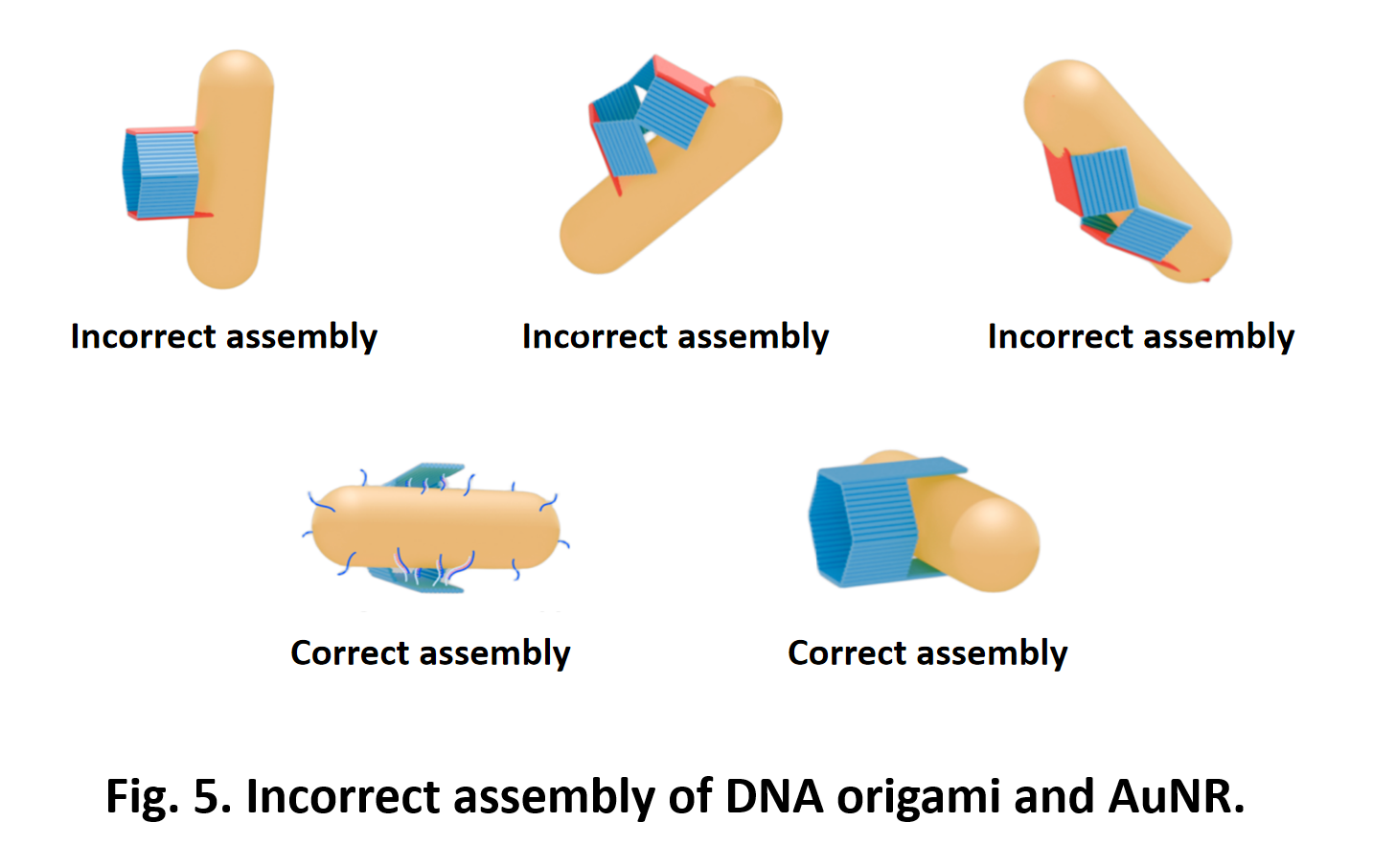
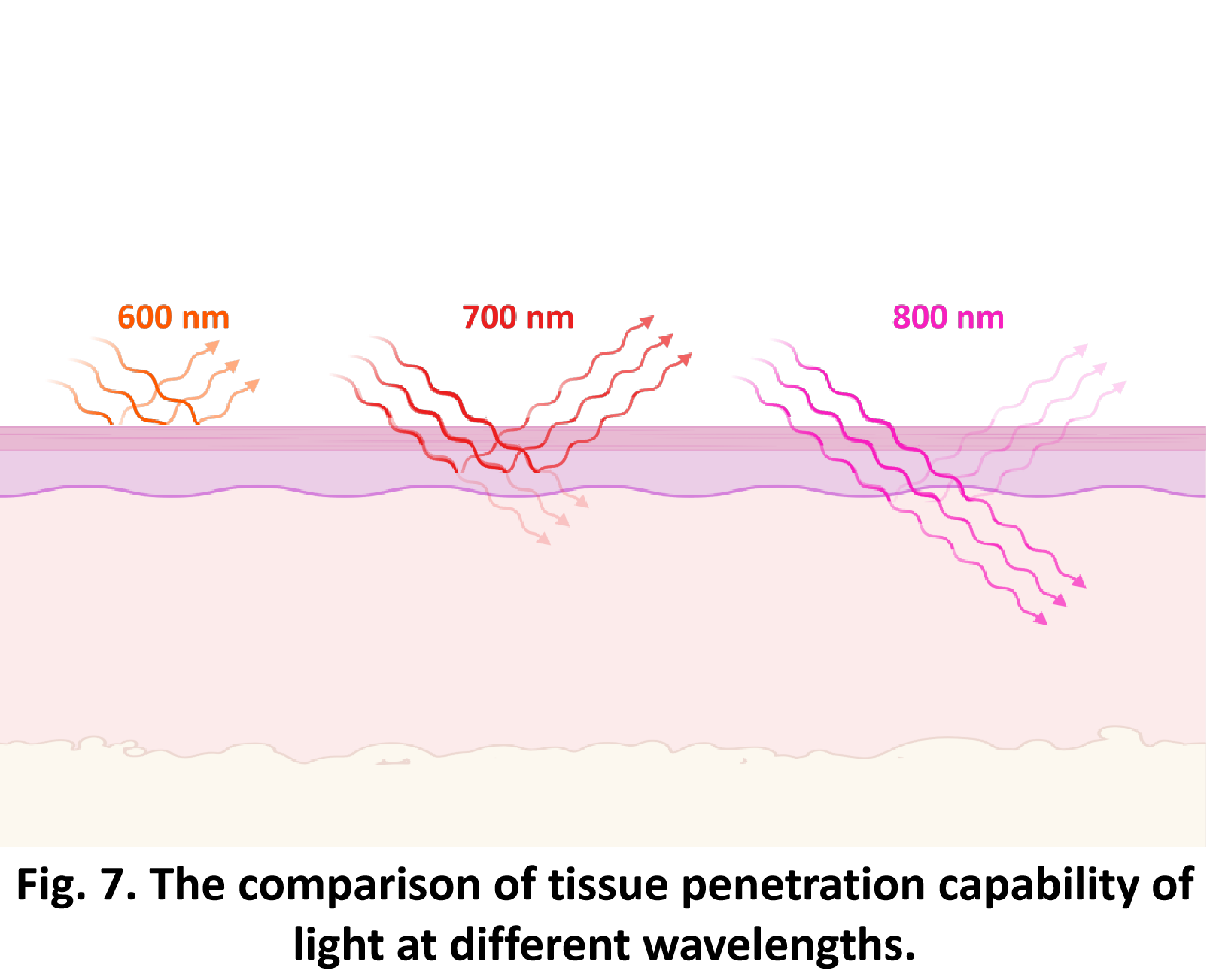
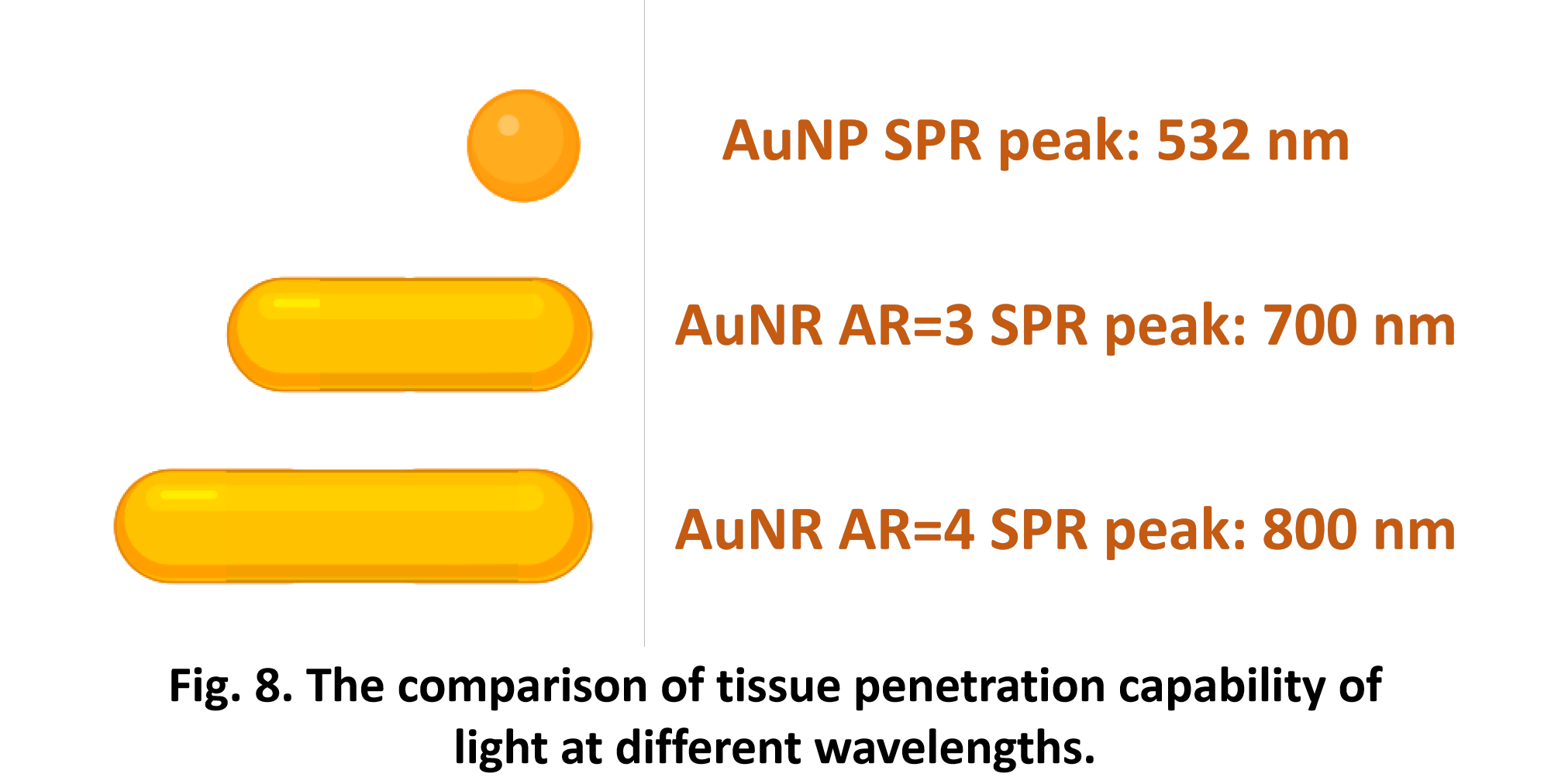
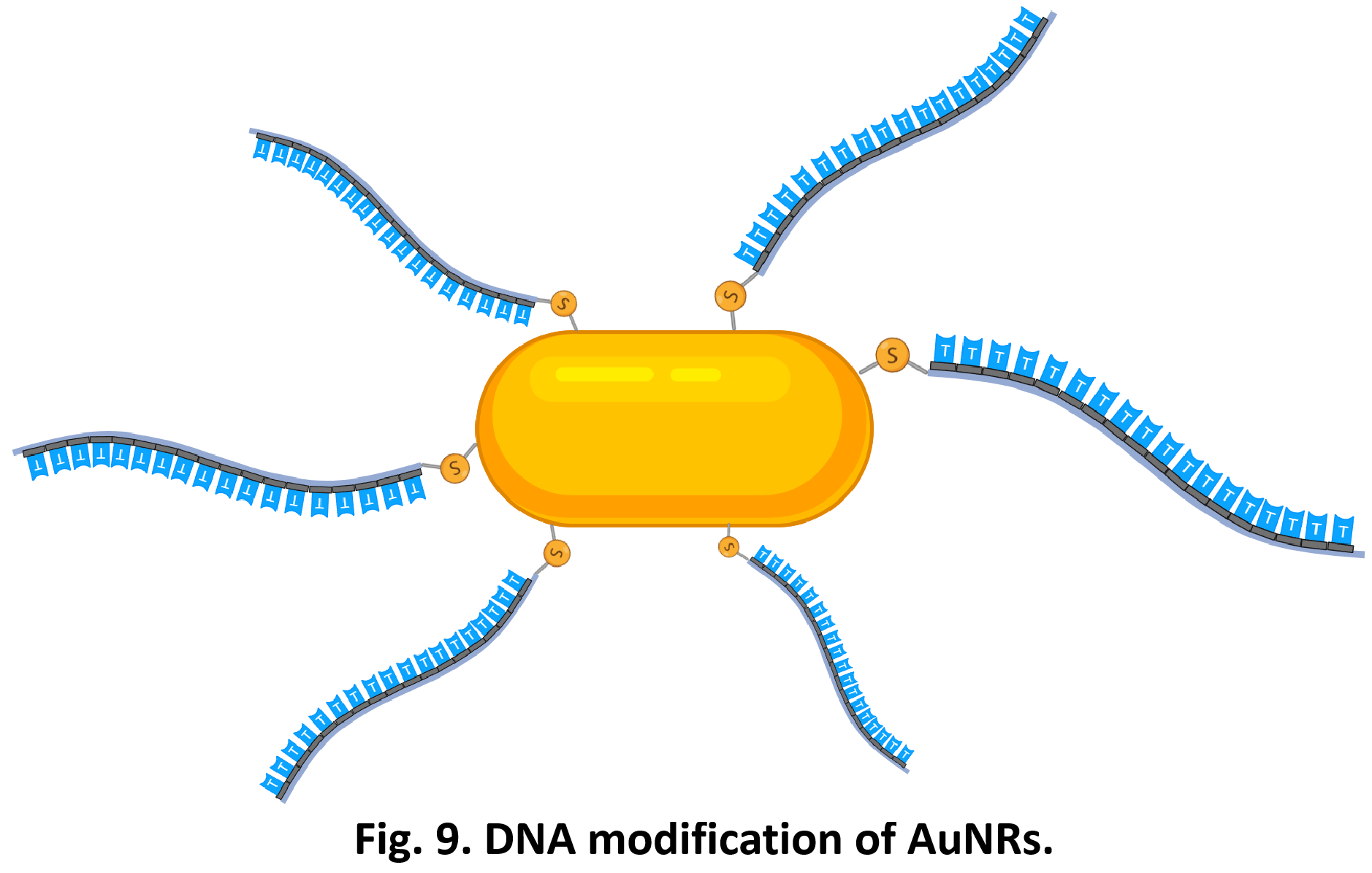
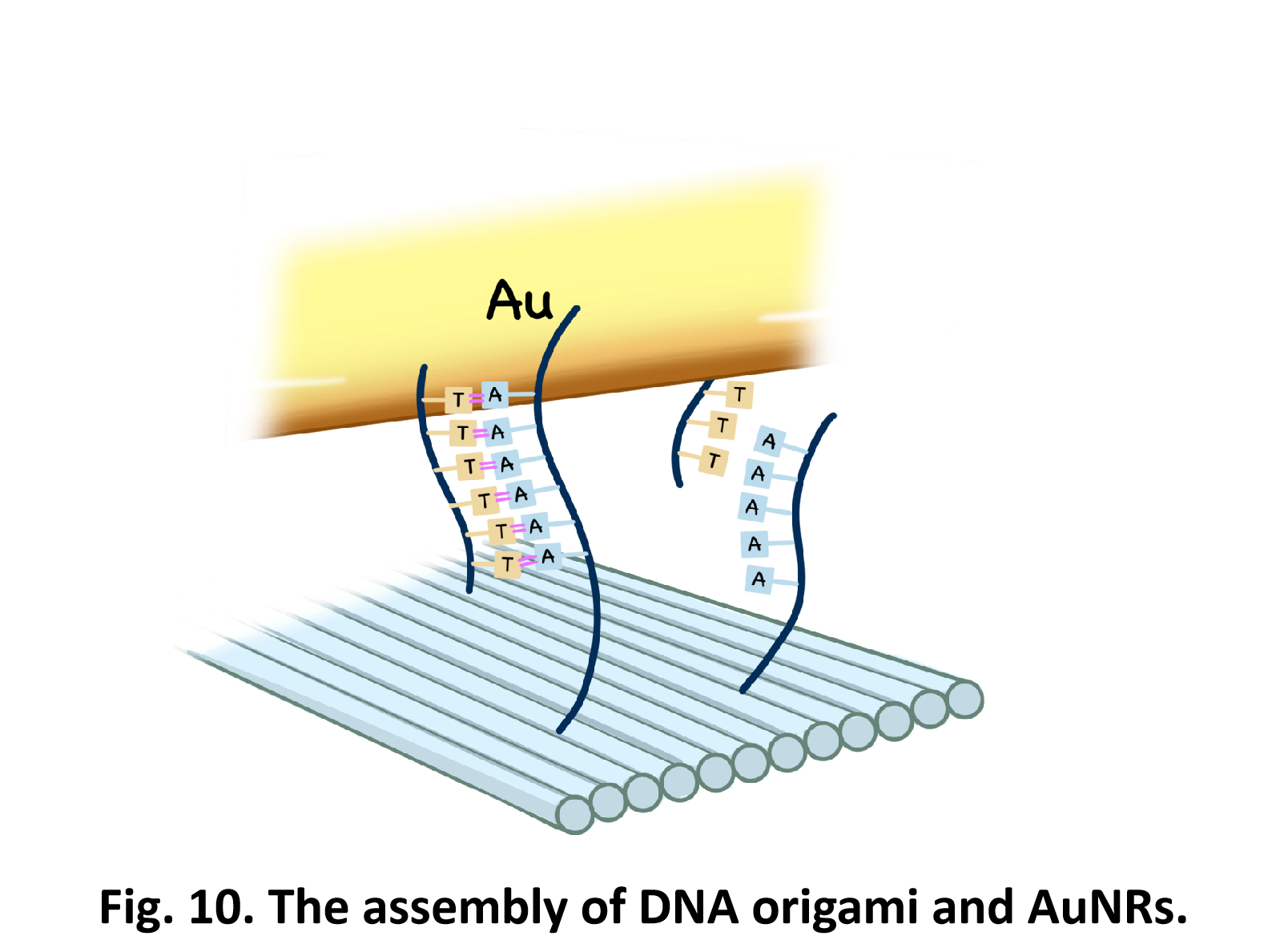
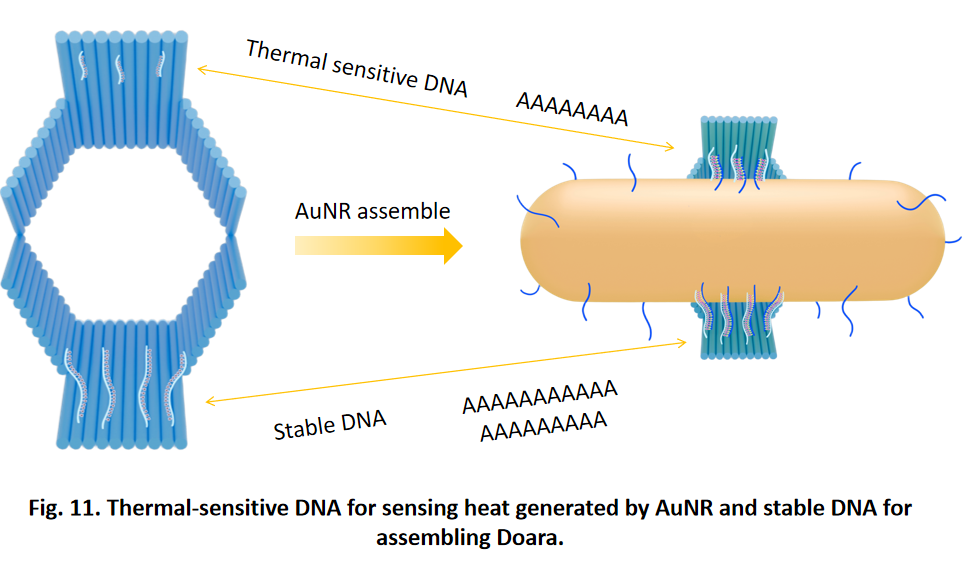
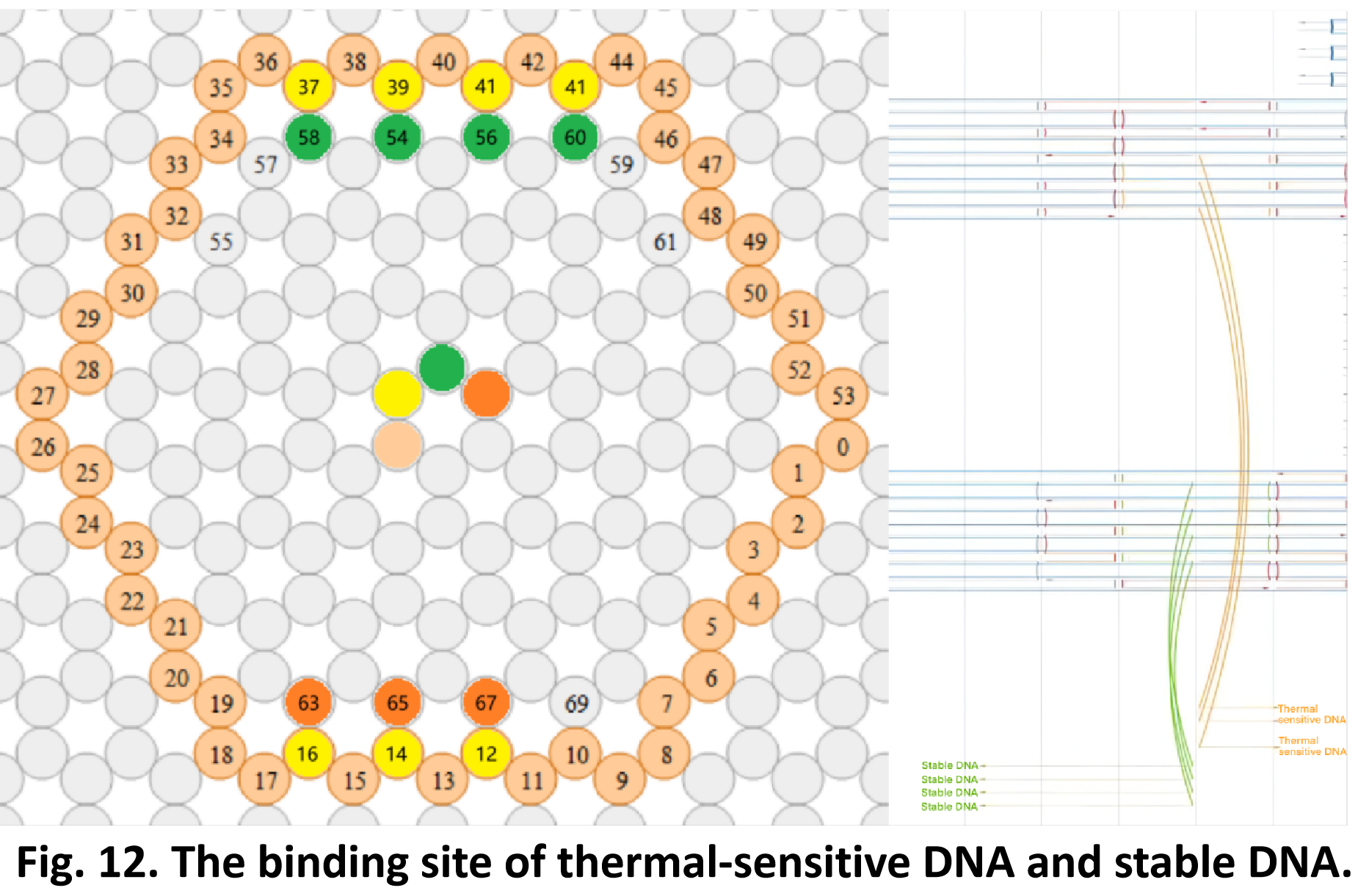
So far, We have successfully assembled Doara. Upon exposure to 808 nm near-infrared light, AuNR initiates surface plasmon resonance, which generates heat and induces the dehybridization of DNA on the AuNR surface. This process results in the opening of the Doara. Once the irradiation is ceased, the DNA on the AuNR rehybridizes, leading to the closure of the Doara. Through this mechanism, we have developed a nanostructure that can be remotely controlled by light, showcasing the potential for light-controlled nanodevices in various applications.
References
[1] Johnson JA, Dehankar A, Winter JO, Castro CE. Reciprocal Control of Hierarchical DNA Origami-Nanoparticle Assemblies. Nano Lett. 2019;19(12):8469-8475. doi:10.1021/acs.nanolett.9b02786.
[2] Oberthür D, Achenbach J, Gabdulkhakov A, et al. Crystal structure of a mirror-image L-RNA aptamer (Spiegelmer) in complex with the natural L-protein target CCL2. Nat Commun. 2015;6:6923. Published 2015 Apr 22. doi:10.1038/ncomms7923.
[3] Chen H, Cong X, Wu C, et al. Intratumoral delivery of CCL25 enhances immunotherapy against triple-negative breast cancer by recruiting CCR9+ T cells. Sci Adv. 2020;6(5):eaax4690. Published 2020 Jan 29. doi:10.1126/sciadv.aax4690.
[4] Douglas SM, Marblestone AH, Teerapittayanon S, Vazquez A, Church GM, Shih WM. Rapid prototyping of 3D DNA-origami shapes with caDNAno. Nucleic Acids Res. 2009;37(15):5001-5006. doi:10.1093/nar/gkp436.
[5] Rothemund PW. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440(7082):297-302. doi:10.1038/nature04586.
[6] Douglas SM, Bachelet I, Church GM. A logic-gated nanorobot for targeted transport of molecular payloads. Science. 2012;335(6070):831-834. doi:10.1126/science.1214081.
[7] Kim DN, Kilchherr F, Dietz H, Bathe M. Quantitative prediction of 3D solution shape and flexibility of nucleic acid nanostructures. Nucleic Acids Res. 2012;40(7):2862-2868. doi:10.1093/nar/gkr1173.
[8] Jabbari A, Sameiyan E, Yaghoobi E, et al. Aptamer-based targeted delivery systems for cancer treatment using DNA origami and DNA nanostructures. Int J Pharm. 2023;646:123448. doi:10.1016/j.ijpharm.2023.123448.
[9] Liu K, Lukach A, Sugikawa K, et al. Copolymerization of metal nanoparticles: a route to colloidal plasmonic copolymers. Angew Chem Int Ed Engl. 2014;53(10):2648-2653. doi:10.1002/anie.201309718.
[10] Zhang X, Servos MR, Liu J. Instantaneous and quantitative functionalization of gold nanoparticles with thiolated DNA using a pH-assisted and surfactant-free route. J Am Chem Soc. 2012;134(17):7266-7269. doi:10.1021/ja3014055.
[11] Chan MH, Chang YC. Recent advances in near-infrared I/II persistent luminescent nanoparticles for biosensing and bioimaging in cancer analysis. Anal Bioanal Chem. 2024;416(17):3887-3905. doi:10.1007/s00216-024-05267-z.
[12] Zhan P, Urban MJ, Both S, et al. DNA-assembled nanoarchitectures with multiple components in regulated and coordinated motion. Sci Adv. 2019;5(11):eaax6023. Published 2019 Nov 29. doi:10.1126/sciadv.aax6023.